Our Strategy
Azura Ophthalmics is utilizing our deep understanding of ocular surface diseases and drug development to deliver a new therapeutic class of Ophthalmic Keratolytics.


Our differentiated approach combines ophthalmologic and dermatologic solutions to harness the unique properties of keratolytics to treat the root cause of numerous underserved ocular indications. Our internally discovered pipeline of new chemical entities allows us to develop a portfolio of first-in-class ophthalmic therapeutics for significant unmet needs.

R&D Pipeline
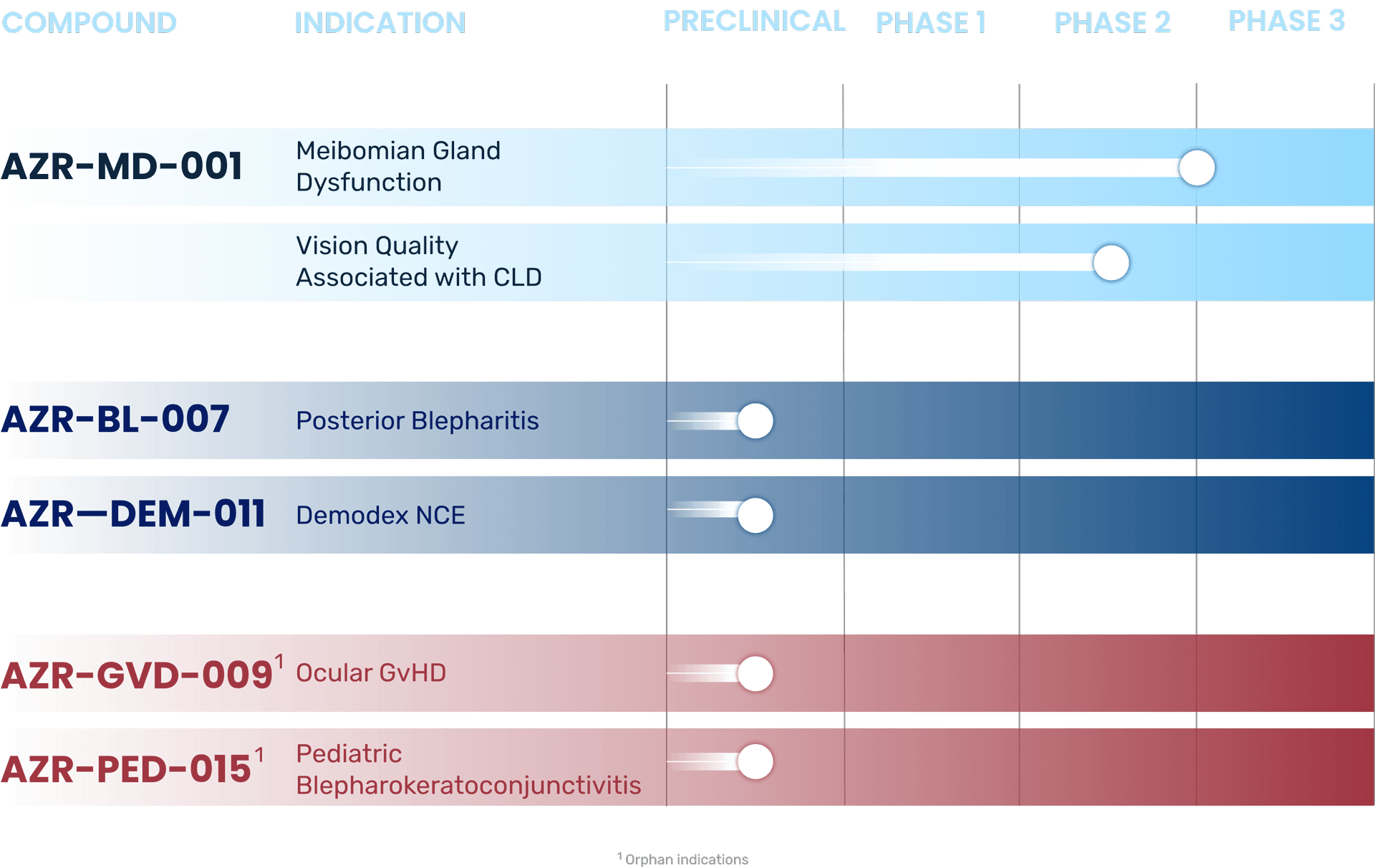


What is Meibomian Gland Dysfunction (MGD)?
MGD is a chronic condition characterized by abnormal keratin production which leads to blocked glands and impacts the quality and quantity of meibum secretions. It is the leading cause of Dry Eye Disease (DED) and Contact Lens Discomfort (CLD). The meibomian glands line the lid margin of the eye.
- Meibum is a complex secretion containing proteins and lipids that help maintain the health of the ocular surface and aqueous properties of the tear film.
- Meibum flows through the ducts and the force of blinking pushes meibum onto the lid border where meibum joins the tear film to coat the eye.
- Even a small disruption in meibum flow can result in inflammatory ocular surface conditions, ocular surface dryness, pain, irritation, and reduced quality of vision.
- If glands remain obstructed, pressure inside of the glands can increase, causing the glands to stop working completely, potentially resulting in irreversible damage to the glands and glandular atrophy.
- Lack of meibum flow results in ophthalmic signs and symptoms of pain, inflammation, irritation, and bacterial infection. 12
- Current treatments for MGD are not sufficient to restore gland function leaving many patients with progressive deterioration in their signs and symptoms as the MGD progresses toward irreversible glandular atrophy.

- Corneal scarring and erosion.
- Scarring of the posterior lid margin.
- Modified ocular surface microbiota.
- Irreversible glandular atrophy.
- Alterations in the normal function of the tear film, which can initiate or exacerbate additional ocular surface diseases such as DED, resulting in corneal ulcers and ocular infections.

AZR-MD-001 harnesses the power of selenium sulfide in an easy-to-use ophthalmic ointment preparation applied directly to the meibomian glands in the lower eyelid
AZR-MD-001 is thought to have a multi-modal mechanism of action that treats the pathophysiology of MGD along with the resulting ocular surface symptoms
- Slows down the rate of keratinocyte proliferation and keratin production, slowing meibomian gland plug formation and obstruction
- Softens keratin aggregates by breaking down disulfide (S-S) bonds, opening obstructions, improving meibum quality and flow through the meibomian glands
- Stimulates lipogenesis to increase the quantity of lipids produced by the meibomian glands
- Kills mites and bacteria on eyelash follicles that can contribute to inflammation and infection
Contact Lens Discomfort (CLD) is a condition where people experience eye pain and irritation during contact lens wear, causing them to reduce or discontinue use of contact lenses.13
The majority of patients with CLD also have MGD.13, 19
The use of contact lenses can disrupt the tear film, and together with mild MGD, patients can experience symptoms of CLD including eye dryness, irritation and an inability to wear contact lenses as desired.13
Removing glandular obstructions in patients with CLD has been shown to normalize the tear film, alleviating the symptoms of CLD.11, 12, 13
It is estimated that more than 40 million adults in the United States, or roughly 17% of the population, wear contact lenses.20 Fifty percent of contact lens users report dry eye symptoms. Studies report that between 12% and 51% of lens wearers “drop out” of contact lens wear, with CLD as the primary reason for discontinuation.12
MGD is one of the most common causes of CLD,21, 22 as many patients with CLD show significant meibomian gland obstruction by built-up keratin, causing the meibomian ducts to become dilated and plugged with thickened meibum.11, 21 The use of contact lenses can stress the tear film, resulting in instability and alterations of the lipid layer, and can cause symptoms to unexpectedly manifest in patients with mild MGD who are otherwise asymptomatic.21, 23
Azura Ophthalmics is currently developing novel treatments for the management of CLD.
clinical Trials

To learn more about, or consider participation in, a current clinical study of one of our investigational therapies, please select the study of interest to be connected to clinicaltrials.gov.
NCT03652051: A Multicenter, Vehicle-controlled, Randomized Study to Evaluate the Safety, Tolerability, Systemic Pharmacokinetics, and Pharmacodynamics of AZR-MD-001 in Patients With Meibomian Gland Dysfunction (MGD) and Evaporative Dry Eye Disease (DED)